In order to be allowed to introduce a medicinal product or vaccine for human use to the market, a pharmaceutical company requires a marketing authorisation.
Rapporteurships and co-rapporteurships assigned to the Netherlands via the centralised procedure (CHMP)
The CHMP is responsible for assessing centralised applications for marketing authorisations for medicinal products. Of the nearly 100 centralised procedures submitted to the EMA in 2022, the MEB served as rapporteur or co-rapporteur in a quarter. 2022 was comparable to 2021, aside from the fact that we served in the role of co-rapporteur more often in 2022 than in 2021. The total number of rapporteurships appears to have decreased slightly over the years; however, the dossiers are usually larger and more complex.
Assigned (co-)rapporteurships
| Co-rapporteur | Rapporteur | |
|---|---|---|
| 2018 | 5 | 18 |
| 2019 | 11 | 24 |
| 2020 | 10 | 19 |
| 2021 | 2 | 21 |
| 2022 | 5 | 17 |
Assigned (co-)rapporteurships
| Country | Number |
|---|---|
| Austria | 16 |
| Belgium | 2 |
| Bulgaria | 0 |
| Cyprus | 0 |
| Czech Republic | 9 |
| Germany | 26 |
| Danmark | 11 |
| Estonia | 5 |
| Greece | 0 |
| Spain | 13 |
| Finland | 5 |
| France | 19 |
| Croatia | 9 |
| Hungary | 7 |
| Ireland | 15 |
| Iceland | 4 |
| Italy | 6 |
| Lithuania | 7 |
| Luxemburg | 0 |
| Latvia | 5 |
| Malta | 1 |
| The Netherlands | 22 |
| Norway | 7 |
| Poland | 11 |
| Portugal | 3 |
| Romania | 2 |
| Sweden | 21 |
| Slovakia | 8 |
| Slovenia | 4 |
In 2022, Germany was assigned the most rapporteurships and co-rapporteurships. The Netherlands was in second place. Sweden, France and Austria rounded out the top five. Four countries were not assigned any rapporteurships or co‑rapporteurships.
More European cooperation
The MEB plays an important role in multinational assessment teams (MNATs). These teams allow a rapporteur position to be performed by multiple countries, giving countries with less experience at serving as a rapporteur an opportunity to gain experience. Of the 22 CHMP rapporteurships and co-rapporteurships assigned to the MEB in 2022, 8 were assigned to MNATs. In most cases, the participating country within the MNAT takes care of assessing the quality aspects of the medicinal product. MNAT partnerships promote European collaboration. They also help the MEB manage its extremely high workload, particularly in the area of quality assessments.
Assignment of pharmacovigilance (PRAC) rapporteurships and co-rapporteurships
The Pharmacovigilance Risk Assessment Committee (PRAC) plays an important role in supervising the risks of medicinal products for people in Europe. The PRAC makes recommendations to the CHMP and CMDh about the risks of medicinal products that have been authorised in the European Union, or are in the process of being authorised.
Assigned pharmacovigilance rapporteurships
| Other | New substances | |
|---|---|---|
| 2018 | 5 | 11 |
| 2019 | 12 | 19 |
| 2020 | 9 | 11 |
| 2021 | 5 | 17 |
| 2022 | 8 | 16 |
In 2022, the MEB was assigned slightly more PRAC rapporteurships than in the previous year.
Of the 24 PRAC rapporteurships assigned to the MEB in 2022, 16 were for new active substances. The remaining rapporteurships related to generics and biosimilars.
Assigned pharmacovigilance rapporteurships
| Country | Number |
|---|---|
| Austria | 7 |
| Belgium | 4 |
| Bulgaria | 0 |
| Cyprus | 0 |
| Czech republic | 2 |
| Germany | 17 |
| Denmark | 9 |
| Estonia | 1 |
| Greece | 0 |
| Spain | 9 |
| Finland | 6 |
| France | 4 |
| Croatia | 5 |
| Hungary | 0 |
| Ireland | 8 |
| Iceland | 0 |
| Italy | 4 |
| Lithuania | 5 |
| Luxemburg | 0 |
| Latvia | 2 |
| Malta | 0 |
| The Netherlands | 24 |
| Norway | 2 |
| Poland | 7 |
| Portugal | 0 |
| Romania | 0 |
| Sweden | 18 |
| Slovenia | 0 |
| Slovakia | 0 |
| United Kingdom | 0 |
With 24 PRAC rapporteurships, the MEB continues to play a leading role at the European level. In 2022, the MEB was assigned the most rapporteurships. The other key players were Germany and Sweden.
At the European level, the MEB is responsible for pharmacovigilance for 101 active substances. This means that for these substances, the MEB is responsible for analysing data in the European database of suspected adverse reactions (EudraVigilance database) and for identifying signals of potential new or changed risks.
Applications initiated via the decentralised procedure (DCP) and mutual recognition procedure (MRP)
The number of initiated procedures for which the Netherlands was the reference member state (RMS) was lower than in 2021.
Applications initiated via the decentralised procedure (DCP) and mutual recognition procedure (MRP)
| MRP | DCP | |
|---|---|---|
| 2018 | 57 | 211 |
| 2019 | 60 | 235 |
| 2020 | 85 | 174 |
| 2021 | 93 | 162 |
| 2022 | 69 | 111 |
Applications initiated via the decentralised procedure (DCP)
| Country | DCP and MRP |
|---|---|
| Austria | 91 |
| Belgium | 1 |
| Bulgaria | 0 |
| Cyprus | 2 |
| Czech republic | 73 |
| Germany | 242 |
| Denmark | 79 |
| Estonia | 14 |
| Greece | 0 |
| Spain | 15 |
| Finland | 38 |
| France | 1 |
| Croatia | 20 |
| Hungary | 37 |
| Ireland | 30 |
| Iceland | 55 |
| Italy | 3 |
| Lithuania | 11 |
| Latvia | 17 |
| Malta | 50 |
| The Netherlands | 111 |
| Norway | 4 |
| Poland | 39 |
| Portugal | 76 |
| Romania | 1 |
| Sweden | 99 |
| Slovenia | 28 |
| Slovakia | 14 |
| United Kingdom | 0 |
Applications initiated via the mutual recognition procedure (MRP)
| Country | MRP |
|---|---|
| Germany | 51 |
| The Netherlands | 69 |
| Sweden | 31 |
| Austria | 16 |
| Denmark | 15 |
| Portugal | 14 |
| Czech republic | 10 |
| Malta | 6 |
| Iceland | 1 |
| Hungary | 11 |
| Finland | 8 |
| Poland | 5 |
| Ireland | 10 |
| Slovenia | 4 |
| Spain | 12 |
| Croatia | 2 |
| Latvia | 1 |
| Slovakia | 3 |
| Estonia | 2 |
| Lithuania | 4 |
| France | 10 |
| Italy | 6 |
| Norway | 4 |
| Cyprus | 3 |
| Belgium | 2 |
| Romania | 0 |
| Bulgaria | 1 |
| Greece | 0 |
| Luxemburg | 0 |
In 2022, Germany was the RMS for the most DCP applications, followed by the Netherlands. The Netherlands was the RMS for the most MRP applications.
Applications via the national procedure
The number of applications submitted via the national procedure (excluding parallel imports, but including duplex procedures) continues to fluctuate between 60 and 80 per year. Most applicants stated a preference for a European procedure (centralised or DCP) so the product could obtain marketing authorisation in multiple European countries at once.
Applications via the national procedure
| 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Number of applications via the national procedure | 57 | 67 | 87 | 59 | 80 |
National parallel import applications
The increase seen in 2021 in the number of applications for a parallel import marketing authorisation did not continue. Fewer applications were submitted in 2022.
Applications for parallel import
| 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Number of applications for parallel import | 521 | 375 | 316 | 486 | 302 |
Number of registered marketing authorisations
In 2022, the total number of registered marketing authorisations in the Netherlands was comparable to the year before.
Number of registered marketing authorisations
| 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Number of registered marketing authorisations | 14288 | 14288 | 13565 | 13452 | 13641 |
Withdrawal of marketing authorisations after registration
If a pharmaceutical company submits a request for withdrawal of a marketing authorisation, the MEB examines whether it relates to a critical product. For example, whether there are comparable products in the same group of medicinal products. If this is not the case, the MEB will explore other possibilities to retain the product for Dutch patients. The MEB does this in consultation with the marketing authorisation holder.
2022 saw a slight decline in the number of withdrawals of marketing authorisations.
Withdrawal after registration
| 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Number of withdrawals | 1209 | 1390 | 1649 | 1150 | 998 |
Scientific advice
By providing scientific advice, the MEB contributes to the responsible development of medicinal products, innovation and early patient access. Scientific advice can concern all aspects of product development, such as clinical or toxicological aspects, as well as regulatory aspects. The advice serves to provide investment security and is a statutory task. There are both national and centralised (European) procedures for providing scientific advice. As a statutory task, providing national advice falls under the direct responsibility of the Board. Centralised scientific advice is prepared by the SAWP and concerns a European consensus under the responsibility of the CHMP. The MEB is active in both European and national scientific advice. Requesting scientific advice is not mandatory.
In 2022, the MEB joined a pilot for simultaneous national advice. This type of advice primarily focuses on clinical studies. The parties concerned can request scientific advice from multiple countries at once.
Assigned scientific advice via SAWP
| 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Number of assigned scientific advices via SAWP | 130 | 142 | 140 | 146 | 140 |
Following the COVID pandemic, the EMA introduced an additional category of scientific advice. Requests in this category are handled by the Emergency Task Force (ETF). In 2022, the ETF issued scientific advice in response to 48 requests. The MEB served as coordinator in respect of 7 of these requests.
Number of national scientific advice requests
The number of national scientific advice requests increased slightly from 2021 to 2022.
Number of national scientific advice requests
| Scientific advice | customised advice (including Covid-19 advice) | |
|---|---|---|
| 2018 | 110 | 6 |
| 2019 | 115 | 14 |
| 2020 | 103 | 10 |
| 2021 | 95 | 6 |
| 2022 | 98 | 12 |
The MEB also gives customised advice, enabling startups, small companies and academic groups to request advice for a reduced fee. This stimulates innovation in universities and small companies. Academic groups were the main parties who contacted the MEB for customised advice in 2022.
Because of the Covid pandemic, a special category of COVID-19 advice was created within the customised advice framework. One request for COVID-19 advice was received in 2022.
As in previous years, the MEB was actively involved in the drug-rediscovery projects of ZonMw. ZonMw is a Dutch institute that stimulates and finances healthcare research and innovation. By assessing project applications for research into medicinal products, which are sometimes already being used off-label in practice, the MEB contributes to potential on-label use. This has important benefits for prescribers and patients, as well as for pharmacovigilance. The registered use forms part of the product information, including the patient information leaflet.
Adverse reactions
Signalling and assessing adverse reactions to a medicinal product, even when the product is already registered, are among the MEB’s core tasks. At the MEB’s request, the Netherlands Pharmacovigilance Centre (Lareb) collects, registers and analyses reports of suspected adverse reactions from medical professionals and patients. Lareb forwards relevant findings from these analyses to the MEB.
In 2022, Lareb and the MEB discussed 63 analyses of reports of suspected adverse reactions. Of these 63 analyses, 34 related to COVID-19,
while 29 concerned other medicines. Of these analyses, 4 items were not analysed further, while 2 items were counted by the MEB as a single item. This explains the difference with the figure reported by Lareb (26 instead of 29).
Of the 63 analyses, 18 were discussed in detail at Board meetings and 4 were discussed by the MEB’s Medical Practice Committee. The other analyses were followed up through ongoing procedures at the MEB or were closely monitored.
Signal detection
At the European level, the MEB is responsible for signal detection for 101 active substances or combinations of active substances. In 2022, the MEB assessed 21 signals in the European context.
The MEB is responsible for the assessment of Periodic Safety Update Reports (PSURs) for the 101 active substances for which it performs signal detection, as well as for all products for which the MEB is the PRAC rapporteur within the PSUR Single Assessment (PSUSA) procedure. A PSUR is a comprehensive and critical analysis of the risk-benefit balance of a product. It provides medicines authorities with an update on global experiences with the safety of a product at set intervals after registration. The submission of a PSUR is determined using a risk-based approach. The frequency with which PSURs must be submitted varies. In 2022, the MEB conducted 128 PSUSA assessments on behalf of Europe.
Direct Healthcare Professional Communication
In the event of urgent and/or important safety issues, healthcare professionals are informed by means of a letter, referred to as a Direct Healthcare Professional Communication (DHPC). A total of 20 DHPCs were sent in 2022. The MEB is exploring the possibility of sending risk communications digitally, including DHPCs and supplementary risk-minimisation material.
Direct Healthcare Professional Communications
| 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|
| Number of DHPC's | 31 | 29 | 22 | 26 | 20 |
Other activities relating to pharmacovigilance
- As in previous years, the MEB and Lareb participated in the MedSafety Week: ‘Working together for the safe use of medicines’..
- The MEB took part in regulatory research with a focus on pharmacovigilance, as it has done in previous years. There were 5 PhD projects in 2022. The MEB and Lareb took part in several European innovation and research projects that were relevant to pharmacovigilance (such as Gravitate Health, UNICOM, ACCESS, Ubiquitous pharmacogenomics and IMI Conception).
- The Patient Information Network was set up, in collaboration with Lareb, doctors’ and pharmacists’ associations and patient groups, to make reliable, accessible and easy-to-understand information available to patients in a simple manner. This network is still active. Results include short summaries of medicine information (Medicine at a glance and Vaccine at a glance). In addition, on 8 December 2022 a declaration of intent was signed by 17 parties in the medicine information landscape, including BOGIN, VIG, VES, Neprofarm, CBD, KNMP, the MEB and the Ministry of Health, Welfare and Sport. The purpose of this collaboration is to develop a new, uniform set of pictograms. The use of uniform pictograms for medicine information contributes to a better understanding, safer and more appropriate use of medicines and greater therapy compliance.
- In 2022, the MEB signed a partnership agreement with the national reporting centre for Preventing Medication Incidents (VMI). The MEB receives information about medication-related mistakes involving unclear or confusing product information or packaging. This information can be used to contribute to preventing incidents.
Medicinal product shortages
The availability of medicines in the Dutch market is extremely important for public health. Last year, the availability of multiple medicines was under threat.
The annual report of the Medicine Shortages and Defects Notification Centre shows that there were more severe shortages in 2022 than in previous years. In addition, there were often situations in which government measures were used to address the shortages. In many cases, these measures were required for long periods.
Over the past few years, the MEB has spent considerable time attempting to stave off imminent shortages. This includes looking for alternatives when potential supply issues arise, medicines are removed from the market or marketing authorisations are withdrawn. The MEB assesses the impact of these events on public health. In 2022, solutions were found for supply issues in most cases. When assessing how critical the temporary or permanent unavailability of a medicine is, the MEB is increasingly noticing that the security of supply of a very small number of alternatives is also under pressure. There are often only one or two alternative marketing authorisations.
Issues can arise simultaneously and accumulate. For example, there was an increase in reports of complex issues at production sites and clinical trial locations affecting the availability of many medicines. In addition, several marketing authorisations for medicines are withdrawn each year, while other medicines are permanently withdrawn from the market for commercial reasons.
These developments are increasingly exacerbating the impact of other threats on the availability of medicines in the Netherlands. For example, the war in Ukraine has caused rising energy and fuel prices, which have put pressure on the availability of resources in the logistics sector. This in turn has led to medicine supply issues.
The MEB will continue its efforts to address imminent shortages in the future. To ensure availability, when changes are made to a registration dossier, for example when a new factory is added, the MEB can prioritise or accelerate the assessment of the changes at the national level. We will also continue to perform tasks for the Medicine Shortages and Defects Notification Centre as efficiently as possible. At the European level, the MEB takes part in discussions focused on reducing the risk of shortages.
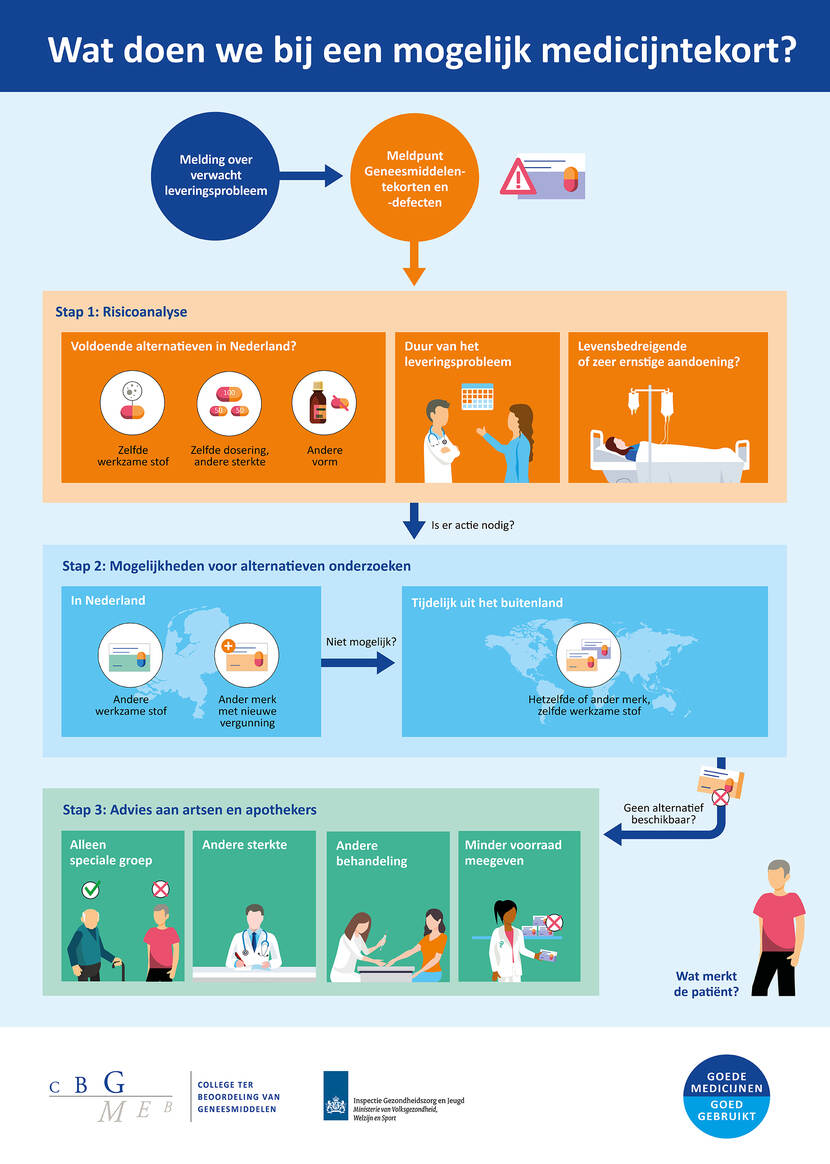
In deze infographic geven we schematisch weer welke stappen we kunnen nemen bij een mogelijk medicijntekort.
Wat doen we bij een mogelijk medicijntekort?
Melding over verwacht leveringsprobleem --> Meldpunt Geneesmiddelentekorten en -defecten -->
Stap 1: Risicoanalyse
- Voldoende alternatieven in Nederland?
- Zelfde werkzame stof
- Zelfde dosering, andere sterkte
- Andere vorm
- Duur van het leveringsprobleem
- Levensbedreigende of zeer ernstige aandoening?
Is er actie nodig? Naar stap 2.
Stap 2: Mogelijkheden voor alternatieven onderzoeken
- In Nederland
- Andere werkzame stof
- Ander merk met nieuwe vergunning
Niet mogelijk?
- Tijdelijk uit het buitenland
- Hetzelfde of ander merk, zelfde werkzame stof
Geen alternatief beschikbaar? Naar stap 3.
Stap 3: Advies aan artsen en apothekers
- Alleen speciale groep
- Andere sterkte
- Andere behandeling
- Minder voorraad meegeven
*In dit hoofdstuk worden kruidengeneesmiddelen en homeopathica buiten beschouwing gelaten. Deze middelen komen in een apart hoofdstuk aan bod: Botanicals en nieuwe voedingsmiddelen.