There are various ways in which a pharmaceutical company can apply for a marketing authorisation for a medicine, namely via the national procedure, the decentralised procedure, or the centralised procedure.
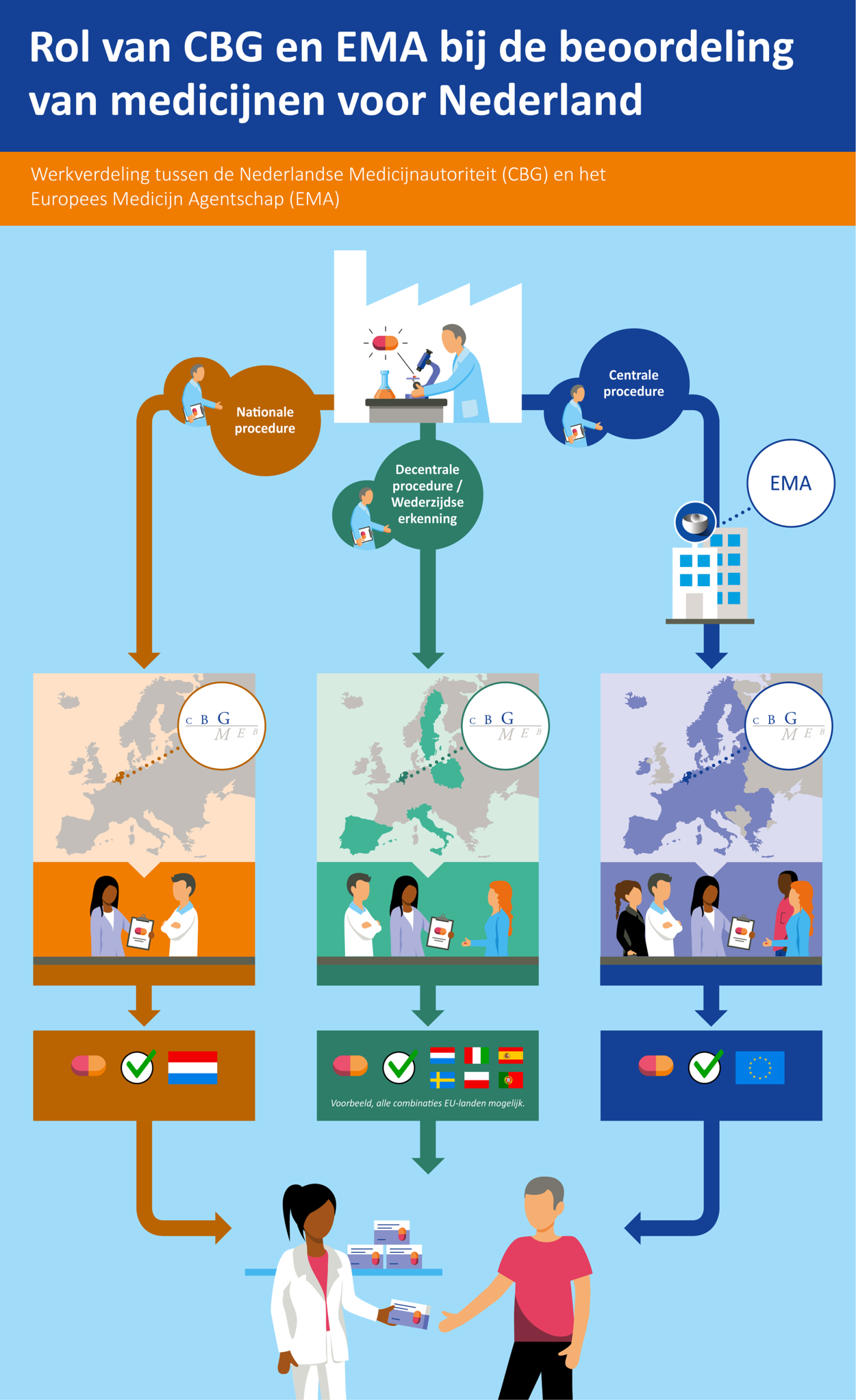
If the national procedure is followed, the company has to submit a dossier to the MEB. The MEB assesses the benefit-risk balance. In the event of a positive opinion, the MEB issues a marketing authorisation which is only valid for the Netherlands. The medicine may then only be marketed in the Netherlands. An application for a parallel import marketing authorisation with a reference to a Dutch reference product is also processed via a national procedure.
The decentralised procedure can be used to obtain a marketing authorisation in several member states, if the company has not yet obtained a marketing authorisation in any European country. The company asks a single country to become a reference member state in the procedure. The medicines authority in that country then leads the dossier assessment. In the event of a mutual recognition procedure the company will already have a marketing authorisation in one country and will want to expand this to other member states. In both these procedures a national marketing authorisation is issued in the event of a positive decision (by all member states involved). The medicine may then be marketed in all the member states involved.
There is also the option of applying for a marketing authorisation which is immediately valid for the entire European Union. This route is referred to as the centralised procedure. It involves a pharmaceutical company submitting the dossier to the European Medicines Agency (EMA). The dossier is assessed by two medicines authorities that then become the rapporteur and co-rapporteur of the Committee for Medicinal Products for Human Use (CHMP). All member states are represented on this committee. After a discussion of the reports which have been drawn up by (co-)rapporteurs after the processing of any comments by other member states, the final judgement of the CHMP is sent to the European Commission for definitive decision-making.
The centralised procedure is obligatory for medicines which have been prepared using biotechnology and for new medicines which are intended to treat, among other things, cancer, AIDS, neurodegenerative illnesses and diabetes because the aim is to be able to offer patients throughout the whole of Europe direct access to these medicines. In the case of other innovative products a company can opt to follow the centralised, decentralised or national procedures.