Photo de Jong Gortemaker Algra

The year in overviewThe following is an overview of events in 2018.
Events

The collaboration in this field of scientific advice is intended to advance the development of medicines and to make these new medicines available to the patient more quickly and easily. Signing by MEB chair Ton de Boer and director of the Dutch Heart Foundation Floris Italianer.

Sufficient women participate in clinical research for medicine authorisation. Sometimes a (small) difference in the effect of medicines on women and men is observed. However, these differences are not so great that women should receive a different dosage. This was the subject of a talk by Dr Christine Gispen (MEB) during the MEB Science Day on 8 February 2018, of which the theme was 'Is drug treatment gender neutral? Gender during the drug life cycle'.

Together with European colleagues and all its partners in the medicines chain, the MEB is continually working to improve the provision and exchange of information. The whole idea is to contribute to one goal, namely: 'Good medicines used better'. During the Provision of Information Day on Thursday 8 March 2018 the MEB exchanged information, thoughts and ideas on this with more than 300 interested parties from pharmaceutical companies, professional organisations and consultancies in the care sector.

The MEB is concerned about the increasing shortage of antibiotics in the Netherlands. The increasing shortage of narrow-spectrum antibiotics means patients are being increasingly given broad-spectrum antibiotics which increase the risk of resistance.

The MEB was established in 1963 after the so-called 'Softenon affair' when it transpired that the use of thalidomide by mothers during pregnancy was extremely damaging for the unborn foetus. Since then strict requirements have applied to the efficacy, safety and quality of medicines.
11 June Envelope with a white or orange hand makes it easier to recognise important risk information

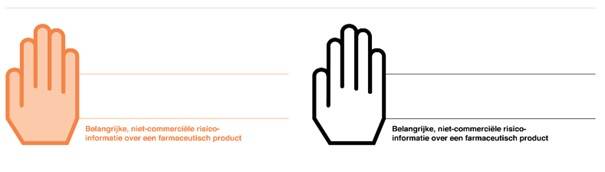
Since 11 June 2018 care providers have been receiving all Direct Healthcare Professional Communications (DHPCs) and additional risk minimisation materials in an envelope bearing a white or orange hand. The orange hand envelope stands for particularly important or urgent DHPCs. All other DHPCs are sent in a white hand envelope.

Innovation and rapid and careful authorisation: during the annual MEB Day the focus was on the possibilities for innovation and the associated challenges.
A number of medicines containing the active substance valsartan are being recalled. This is due to them containing an undesirable substance (NDMA).

Prof. R. J. (Rob) van Marum was sworn in as a new member of the MEB on 5 July 2018. As a clinical geriatrician and clinical pharmacologist, he is affiliated to the Jeroen Bosch Hospital in 's Hertogenbosch. He is also a professor by special appointment in 'Pharmacotherapy among the elderly' at the Department of General Practice and Geriatric Medicine at the Amsterdam UMC.
Vera Deneer was appointed vice-chair of the MEB in December. She has been a member of the MEB since June 2015. Vera Deneer shares the position of vice-chair with member Prof. Dr Pieter de Graeff.

The MEB was given a greater role in various European medicine committees. In 2018 Dr Sabine Straus was elected as chair of the European Pharmacovigilance Risk Assessment Committee (PRAC) in London. Dr Violeta Stoyanova-Beninska was elected as new chair of the committee for orphan medicines of the European Medicines Agency. Ms. Kora Doorduyn was elected as the new vice-chair of the CMDh (Coordination Group for Mutual Recognition and Decentralised Procedures).

Huge numbers of manufacturers of over-the-counter medicines have responded to the appeal by the MEB to state the shelf life after opening on the packaging. It is estimated that, in 2015, only 10% of over-the-counter medicines with resealable packaging, such as bottles of cough mixture, had this important information.
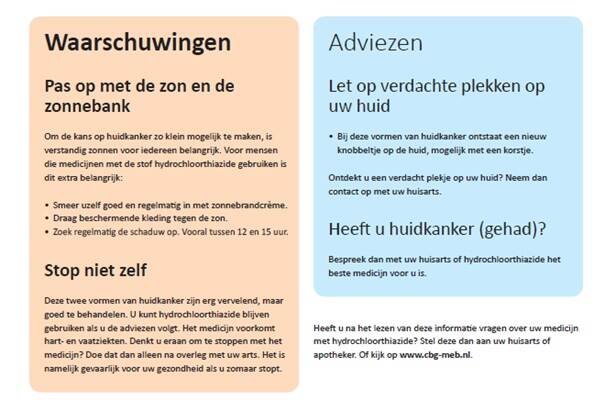
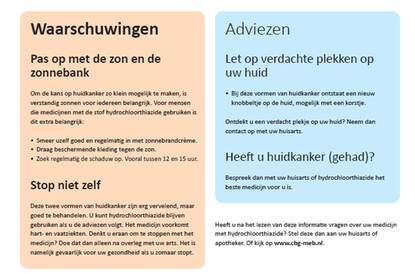
Research has shown that patients who use hydrochlorothiazide (HCT) for a long period of time run an increased risk of two types of skin cancer, namely basal cell carcinoma and squamous-cell carcinoma. Hydrochlorothiazide makes the skin more sensitive to damage by UV radiation from sunlight and sunbeds. Patients who use this medicine are therefore advised to take a responsible approach to exposure to UV radiation. With this in mind, and in consultation with doctors, pharmacists and patient representatives, the MEB made an information card for patients.

Patients need reliable and comprehensible information on medicines that is easier to find online. To achieve this Minister Bruno Bruins (VWS) launched the Patient Information Network. This network consists of the MEB, Lareb, the Royal Dutch Pharmacists Association [Koninklijke Nederlandse Maatschappij ter bevordering der Pharmacie] (KNMP), the Dutch College of General Practitioners [Nederlands Huisartsen Genootschap] (NHG), Netherlands Institute for Health Services Research [Nederlands instituut voor onderzoek van de gezondheidszorg] (NIVEL), the Dutch Centre of Expertise on Health Disparities (Pharos) and the Dutch patients' Federation [Patiëntenfederatie Nederland].

With this in mind Stichting Kijksluiter and the MEB signed an agreement at the Ministry of Health, Welfare and Sport.

In practice there is regularly confusion and dissatisfaction about switching medicines. Patients indicate that, in particular, frequent switching leads to unnecessary (health-related) problems. That is why the Ministry of VWS has instructed a working group to start drawing up as comprehensive a list as possible of medicines which should preferably no longer be switched. The working group is made up of representatives from patient organisations, doctors, pharmacists and insurers. Prof. Ton de Boer, chairman of the Medicines Evaluation Board (MEB) is leading the working group. The working group is expected to present its results in May 2019.