The year 2021 was a year packed with memorable moments. It was a year that saw the number of COVID-19 vaccines reach 5, plus the introduction of a number of medicinal products for the treatment of COVID-19. But it didn’t stop there. The year in overview.
The MEB had a high media profile in 2021. During press conferences and live broadcasts to the general public, such as the ‘Grootste Coronaspreekuur: Vragen over Vaccins’ (‘Great COVID Consultation Hour: Questions about Vaccines’). A panel of MEB employees helped behind the scenes to give viewers the correct answers.
Online
The pandemic meant that many events took place primarily online. The same applied to the MEB Science Day and MEB Day. Both events were held online for the first time. Contact with marketing authorisation holders of veterinary medicinal products regarding the implementation of the new Veterinary Medicinal Products Regulation also took place online.
The general public does not have a problem with digital information, as also demonstrated by the statistics for the MEB’s websites and social media channels. Our social media reports received more than three million views. CBG-MEB.nl was visited almost 6.7 million times in 2021. Placing the website in the top 15 of all (1,750) central government websites. Our Medicines Information Bank website Geneesmiddeleninformatiebank.nl also features high on this list (with nearly 5 million visits).
The year in overviewAn overview of the most important events in 2020.
Events

In January, the European Medicines Agency (EMA) assessment committee issued a positive recommendation on two COVID-19 vaccines. Another two followed later in the year.

Experts Diederik Gommers, Marjolein van Egmond, Agnes Kant and Ton de Boer had their say during the ‘Great COVID-19 Consultation Hour’ organised by the MEB.

The theme of the online Science Day 2021 was the evolution of Pharmacovigilance over 25 years.

Paula Loekemeijer became the new Directory/Secretary of the MEB Agency on 1 May 2021. She succeeded Hugo Hurts, who retired on 1 September 2021.

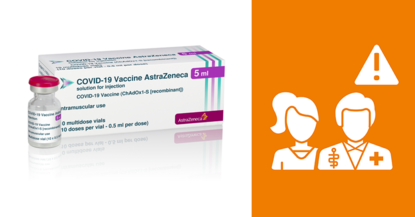
There is potentially a very low risk of severe clot formation and reduced blood platelet count after receiving a dose of the AstraZeneca COVID-19 vaccine. The warning to be alert to this risk was included in the product information.

The MEB has produced a list of medicinal products for which substitution, in the interest of the patient, is undesirable. These are products whereby incorrect use leads to serious problems. The list was commissioned by the Ministry of Health, Welfare and Sports.

The cooperation agreement between the drugstore association CBD and the MEB was signed on 8 April. This date marked the start of a collaboration to ensure the proper use and availability of OTC medicinal products.

Research carried out by the MEB shows that medicinal products are not only tested on males, as it is sometimes believed. In all 9 disease areas studied, females were included in drug trials.
The MEB presented its Science Policy 2020-2024. Under the title ‘Regulating with the knowledge of tomorrow’, eight main themes were identified that will form the basis for shaping policy on regulatory research in the years to come.

The Medical Device Regulation entered into force in May 2021

The Pfizer/BioNTech (Comirnaty) COVID-19 vaccine was the first coronavirus vaccine to receive a positive recommendation for use in children aged 12 and over. The vaccine had already received conditional approval and was being used in people aged 16 and over. This was followed in July by a positive recommendation for Spikevak (the Moderna COVID-19 vaccine) in children aged 12 and over.
The MEB organised a number of digital sessions on the use of pictograms. A public consultation was also held. This is the start of a broader exploration of the use of pictograms.

During the ‘Junior COVID-19 Lecture: Questions about Vaccines’, Ton de Boer, ethicist/philosopher Naomi van Steenbergen and paediatrician Patricia Bruijning answered questions from secondary school pupils about COVID-19 vaccines. This event was organised in response to the Health Council of the Netherlands’ recommendation that healthy children aged 12 and over should also be offered a COVID-19 vaccine.

A number of medicinal products for the treatment of high blood pressure and heart failure were found to contain an impurity. The affected medicinal products were those containing losartan, valsartan or irbesartan. The manufacturers of these medicinal products issued a recall for non-compliant batches. This took place in consultation with the Health and Youth Care Inspectorate and the MEB. The majority of medicinal products tested did not exceed the limit and did not need to be recalled. There was no direct risk to patients.

Prof. Ton de Boer was reappointed Chairman of the Medicines Evaluation Board. This marked the start of his second four-year term as MEB Chairman.

The Veterinary Medicinal Products Unit organised a number of online information meetings to answer questions about the Veterinary Medicinal Products Regulation. The first took place on 28 September. The meetings were well attended.

Hugo Hurts, Director/Secretary of the Medicines Evaluation Board until 1 May 2021, has retired. He looked back on his 36-year career in central government. “Seeking a balance, working together and looking beyond the horizon.”
European-level recommendations were made on a booster or third jab of a COVID-19 vaccine.
The notification form of the Medicine Shortages and Defects Notification Centre has been made more user-friendly. Various stakeholders were involved in the process, including marketing authorisation holders who submit notifications.

International MedSafetyWeek is an annual campaign in which the Medicines Evaluation Board and Netherlands Pharmacovigilance Centre Lareb take part. The aim of this week is to raise awareness about reporting adverse reactions. The theme of this year's MedSafetyWeek was vaccines.

More than 600 interested parties actively took part in the online MEB Day 'Balance in turbulent times' on 10 November. Under the guidance of chairman of the day Tom van ‘t Hek, we looked back with speakers from the medical, pharmaceutical and administrative field on an eventful six months. We also looked ahead to the future.

On 25 November, the Pfizer/BioNTech (Comirnaty) COVID-19 vaccine received a positive assessment for use in children aged 5 and over. The vaccine consists of two low-dose jabs administered 21 days apart.
There was a great deal of activity surrounding a range of COVID-19 medicines and vaccines in December. Various monoclonal antibodies were approved for the treatment of specific patients with COVID-19. The antiviral drug remdesivir can be prescribed for a larger number of patients.
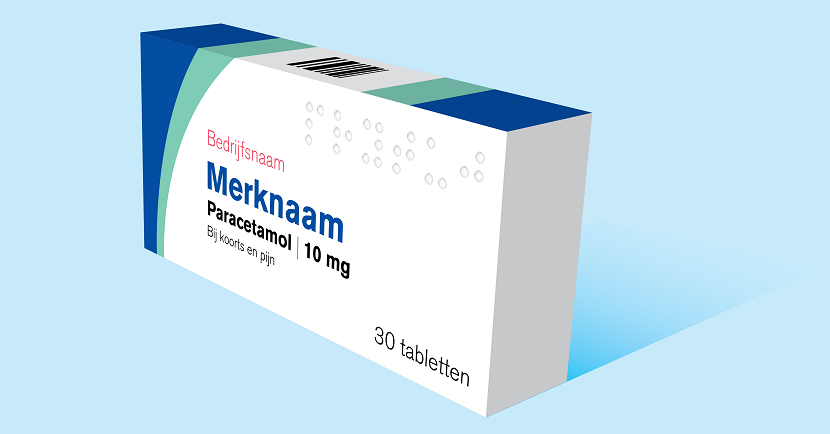
The Board approved the pictogram policy following a public consultation. Our policy documents have been updated. Pictograms can now be used on the outer packaging of medicinal products.