2021 was the second year to be largely dominated by the COVID-19 pandemic. Thanks to hard work and intensive European cooperation, several COVID-10 vaccines and medicines were assessed for efficacy, quality and safety.
Many procedures
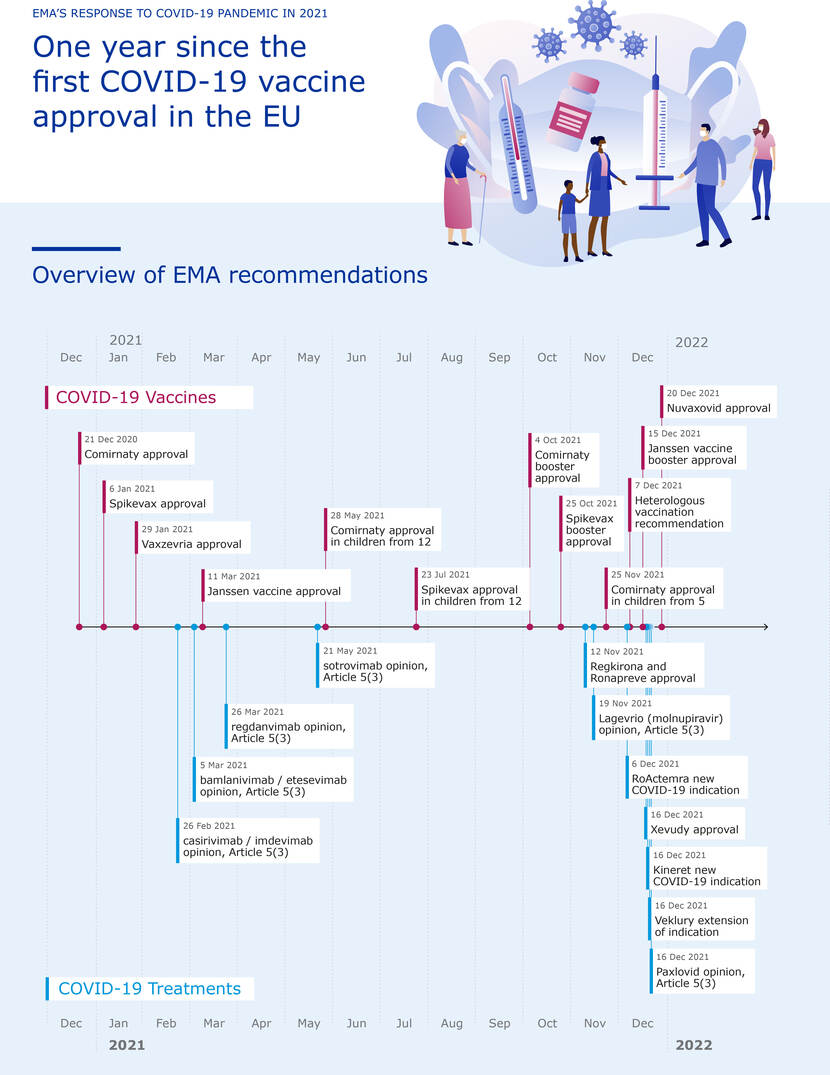
By the end of 2021, five COVID-19 vaccines were available in Europe with a conditional or unconditional marketing authorisation. There are also four medicines that can each be used in their own way for the treatment of COVID-19. For two medicines that were already authorised for a different indication, the manufacturers have submitted data for the treatment of COVID-19. All of these medicines and vaccines were largely assessed in 2021. However, safety continues to be closely monitored even after marketing authorisation has been granted. From collecting and analysing reports of suspected adverse reactions, identifying potential risks, to going through new data, such as scientific literature, studies and follow-up studies provided by the manufacturer.
The conditional and unconditional marketing authorisations for the COVID-19 vaccines, in a number of which the Netherlands was also involved as rapporteur or co-rapporteur, entailed a lot of work in a variety of ways. Vaccines and medicines were assessed via an accelerated procedure: the ‘rolling review’. In this procedure a company shares information with the medicines authority while clinical research is still ongoing. This makes it possible for the assessment to start earlier and saves a lot of time. The rolling review is an intensive approach with very short deadlines, which is not sustainable in this form in the longer term. The procedures have placed high demands on the MEB’s assessors and Regulatory Project Leaders.
On top of this, numerous variations on the marketing authorisations were submitted and assessed. For example, over the course of the year the indications were expanded for Comirnaty and Spikevax, the BioNTech/Pfizer and Moderna COVID-19 vaccines, to include children and young people aged 12 and over (Comirnaty and Spikevax) and later 5 and over (Comirnaty). The indication for use of the COVID-19 medicine Veklury (remdesivir) was also expanded.
Additional efforts were made in relation to pharmacovigilance in 2021. As the PRAC Rapporteur for Comirnaty, the Netherlands plays a leading role in signal detection and the assessment of protocols and results of post-marketing studies in a European context. The MEB works closely with the National Institute for Public Health and the Environment and the Netherlands Pharmacovigilance Centre Lareb, as well as with other institutions at European level. Reports of suspected adverse reactions of COVID-19 vaccines from both Lareb and the companies’ periodic safety update reports are assessed on a monthly basis.
Procedures for which the Netherlands was rapporteur or co-rapporteur generated a considerable amount of work. The same was true of the procedures in which the Netherlands was involved as a ‘concerned member state’.
Despite the large number of procedures and short deadlines, the MEB gained more experience of coordination with the Board outside of Board meetings. The expertise of MEB members is also widely used outside of meetings. Thanks to input from members of the Medicines Evaluation Board, decision-making remained at a high level.
A legible and larger version of this infographic can be downloaded here.
Communications
COVID-19 vaccines received more media attention than any other topic in the last year. As a result, the MEB was also firmly in the spotlight. As a source of reliable, scientific, independent information, the MEB therefore actively sought out the media in order to answer questions. One of the ways we did this was through press conferences. A logical, but new step in the history of the MEB. In the end, we held eight press conferences in 2021.


Consumers and patients also increasingly turned to the MEB for information on COVID-19, the vaccines and medicines. More than 1,000 direct questions about COVID-19 were submitted using the contact forms on the website in 2021, and we also responded to numerous questions on social media. During live broadcasts on YouTube, experts answered questions about the COVID-19 vaccines:
- 31 January: the Great COVID Consultation Hour – Questions about Vaccines
Under the guidance of presenter Merel Westrik, Diederik Gommers (NVIC), Professor of Immunology Marjolein van Egmond (Amsterdam UMC) and MEB Chairman Ton de Boer answered questions about COVID-19 vaccines. The programme was broadcast live on YouTube and on SBS6. - 12 July: ‘Junior COVID-19 Lecture: Questions about Vaccines’
Chairman Ton de Boer, paediatrician/epidemiologist Patricia Bruijning (UMC Utrecht) and ethicist Naomi van Steenbergen (Utrecht University) answered questions from secondary school pupils about COVID-19 vaccines. The lecture was broadcast live online and was attended by pupils on location.
Information that is both reliable and understandable for everyone
The Junior COVID-19 Lecture and the COVID Consultation Hour responded to the needs of the public by providing answers to the most frequently asked questions about COVID-19 vaccines and medicines. As a supplement to the official patient information leaflet, we worked with the National Institute for Public Health and the Environment to develop a ‘Vaccine in Short’ leaflet for each COVID-19 vaccine. This is a single A4 page with easy-to-understand information about the advantages and disadvantages of the vaccine. It is a new, visual and short form of vaccine information, available via a number of channels including the MEB websites and coronavaccinatie.nl. In 2021, the ‘Vaccine in Short’ leaflet for SpikeVax and Comirnaty was also distributed and handed out at a large number of vaccinations sites, in collaboration with the National Institute for Public Health and the Environment and the Municipal Health Services. The 'Vaccine in Short for Children' leaflet was also enclosed with the COVID-19 vaccination invitation for children aged between 5 and 11.