Scientific basis
Science is the driver behind nearly everything that the MEB does. Science resonates in the assessment of medicine dossiers, in the decision whether a medicine should be granted a marketing authorisation, how medicine safety can best be monitored, and how medicines will ultimately be used in practice. The MEB spends around 3% of its budget on research, of which the importance and progress is monitored by the Science Committee (see Appendix D for the composition of this committee). Research conducted by the MEB falls under three primary pillars: development and innovation, regulation and decision-making, and safety and consumer use. An overview of the research projects currently being conducted by the MEB is provided in the leaflet ‘The MEB Regulatory Science Programme 2016 and beyond’, which was published in 2016 and which lists the research projects and collaborating academic centres. This information can also be found on the MEB website.
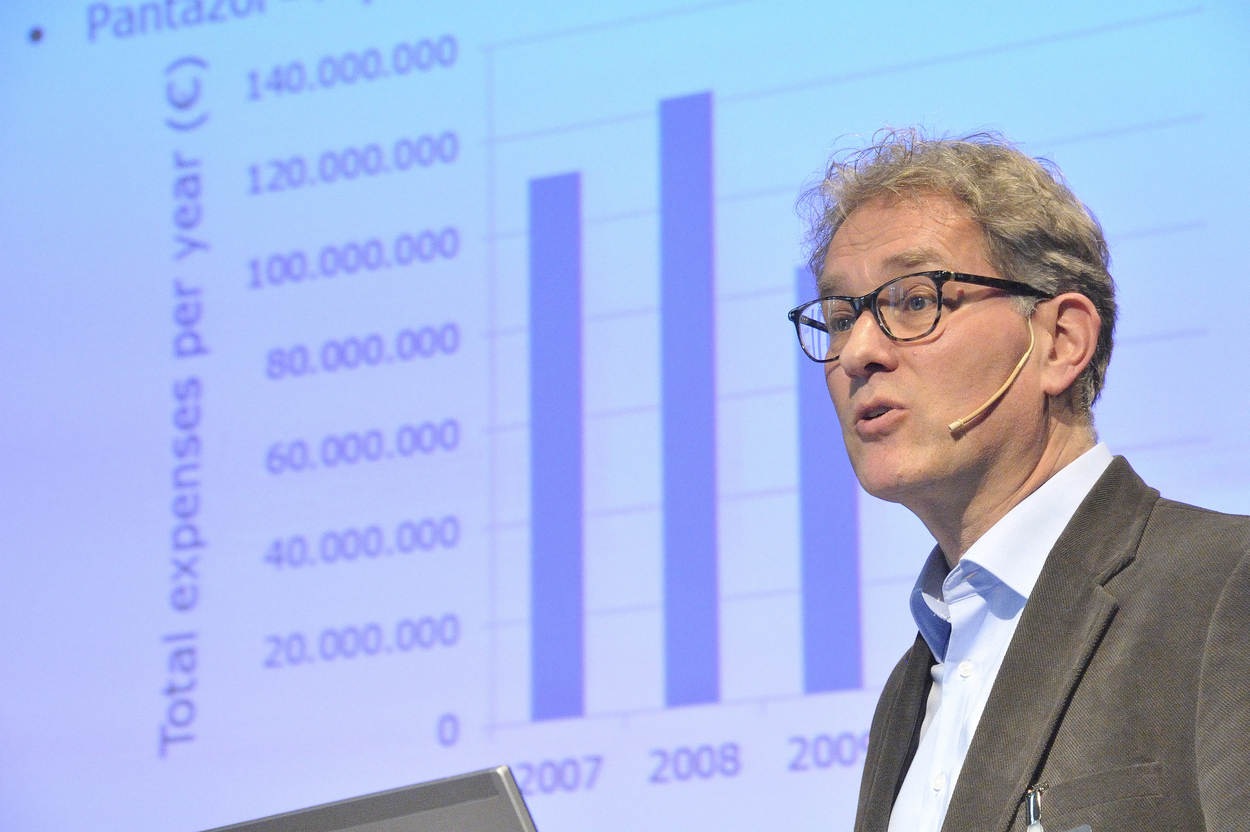
The theme of the sixth edition of the MEB Science Day was ‘Generic medicines in society: The dilemma of the regulator’. A generic medicine is equivalent to a medicine that has already been approved earlier (the reference medicine or innovator product) of which the patent protection period has expired and of which the bioequivalence with the reference medicine has been demonstrated in relevant studies. During the day, attention was paid to the doubts among prescribers regarding the reliability of generic medicines, in spite of the fact that these medicines have been assessed and authorised. Recent PhD research by Abby Yu also confirms that there is very little reason to doubt the bioequivalence of various medicinal products. An important conclusion, certainly also because the costs for generic medicines are a fraction of those of branded medicines. The MEB pays constant attention to patients who report complaints when switching from a branded medicine to a generic medicine, certainly when it concerns medicines for which it is difficult to determine the exact correct dosage. It is essential for MEB to provide good and clear information to doctors, pharmacists and users. In 2017 the MEB published a leaflet with answers to questions about generic medicines for both consumers and healthcare providers.
An account of the Science Day has been published in the MEB Regulatory Science Magazine. The first edition of this magazine was launched at the Science Day 2017. The Regulatory Science Magazine is intended for everyone who is interested in how science influences and changes the regulatory field. The MEB and Dutch researchers play a prominent role in this.
A summer workshop was organised by the Regulatory Science Network Netherlands (RSNN) in 2017. The MEB participated in this. The workshop was about clinical endpoints and how these can be improved. Patients, regulators, researchers and the pharmaceutical industry were brought into contact with each other and discussed possible solutions.
The RSNN organised a symposium at the FIGON Dutch Medicine Days on 2 October 2017. The theme was early diagnosis of diseases and how medical intervention can contribute positively in this early stage. The MEB contributed to the annual symposium of The Organisation for Professionals in Regulatory Affairs (TOPRA) in London on 3 October in a Regulatory Science session. The RSNN, Centre for Innovation in Regulatory Science (CIRS) and the EMA also participated in this symposium.
With a scientific symposium, programme manager science, the MEB said goodbye to Dr. Christine Gispen on 1 November 2017. She made a big contribution to the further development of MEB's scientific programme. You can read more about this in the Regulatory Science Magazine, for example in the interview with Hans Georg Eichler, Senior Medical Officer at the EMA.